
Promoting VeriSee Dr AI device jointly by Acer Medical and Yung Shin Pharmaceutical to extend patient access.
Latest News
2023 / 05 / 19
Acer Medical (6857) announced on May 18th to partner with Yung Sin Pharmaceutical Group (3705) that both signed a memorandum of understanding (MOU) to jointly promote Acer Medical's smart medical device, VeriSee DR (AI-assisted diagnosis of diabetic retinopathy). By combining Yung Sin Pharmaceutical's resources in the pharmaceutical sector and its experience in diabetic care, the companies aim to accelerate the patients' easy access of smart healthcare in Taiwan. This move will also help medical institutions improve the efficiency of diabetic retinopathy screening and enhance the quality of care for diabetic patients.
Acer Medical's General Manager Yin-Hsong Hsu stated, "The alliance between Acer Medical and Yung Shin Pharmaceutical will create synergies in both parties' AI technology and pharmaceutical expertise. Together, we will promote the use of AI-assisted diagnostic software, VeriSee DR, in clinical diagnosis, benefiting more diabetes patients. Acer Medical aims to fully integrate artificial intelligence into the medical field, further accelerating the productization and commercialization of smart healthcare, and helping medical professionals more effectively deliver health care, disease prevention, and improved medical quality."
Barbara Lee, General Manager of Yung Shin Pharmaceutical, stated, "Yung Shn Pharmaceutical and Acer Medical have collaborated to provide Taiwan's medical community with rapid diabetic eye screening through their respective expertise. This not only allows diabetic patients to quickly become aware of their conditions through AI technology, but also assists physicians in diagnosis and helps them manage their patients' blood sugar levels. This enables value maximization of smart healthcare, enhances communication between doctors and patients, and helps patients maintain stable health."
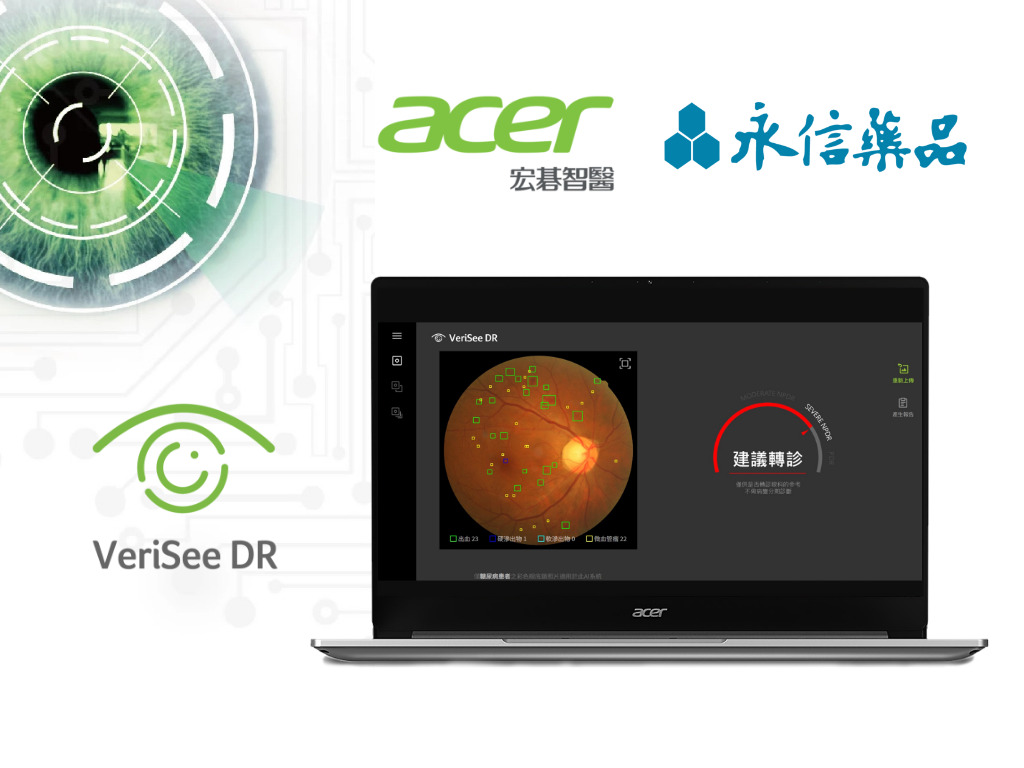
Diabetic retinopathy (DR) is a common complication of diabetes and a leading cause of blindness in adults. The "2022 Clinical Care Guidelines for Type 2 Diabetes" published by the Diabetes Association of the Republic of China recommends that patients with diabetes undergo at least annual visual acuity and fundus examinations with dilated pupils to monitor their eye condition. In 2022, the national average diabetic retinopathy screening rate was only 45.53%, and more than half of diabetic patients still do not undergo regular follow-up. Early screening, diagnosis, and appropriate therapeutic interventions are crucial for protecting the vision of patients with diabetes.
Acer Medical's VeriSee DR is the first smart ophthalmic medical device in Taiwan to receive a license from the Ministry of Health and Welfare's Food and Drug Administration (TFDA). Since its launch in September 2020, it has been introduced in over 160 medical institutions at all levels, and its AI fundus screening service is estimated to benefit over 350,000 diabetics annually. VeriSee DR utilizes artificial intelligence and deep learning technologies to analyze fundus images, providing AI-powered interpretation suggestions and disease annotations. This helps diabetes physicians save time, improve interpretation accuracy, and promptly refer high-risk diabetics to ophthalmologists for follow-up treatment, further reducing the risk of blindness.