Yung Shinn Pharmaceutical invests in the clinical development of a new drug for acromegaly
Latest News
2020 / 01 / 16
Yung Shin Pharmaceutical (Taichung, Taiwan) (hereinafter referred to as Yongxin Pharmaceutical) and GlyTech, Inc. (Kyoto, Japan) (hereinafter referred to as GlyTech) have agreed to collaborate on the first clinical development of glycosylated somatostatin (G-SRIF) in Japan in early 2020. G-SRIF was initially developed as a pharmaceutical product by GlyTech and Nippon Shokubai Co., Ltd., a major Japanese chemical company, in 2015. Yung Shin Pharmaceutical is a new member of the drug development team in this collaboration.
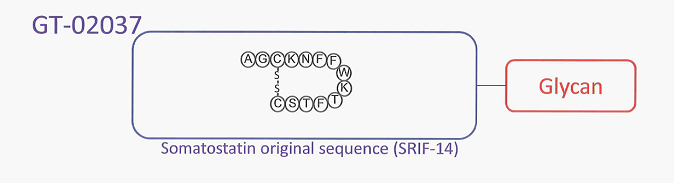
Somatostatin, a peptide hormone, inhibits the secretion of growth hormone in the human body. The first-generation somatostatin (Octreotide, trade name: Sandostatin), created by inserting artificial amino acids, has a high affinity for the second subtype of the somatostatin receptor and is a best-selling drug with annual revenue of 150 billion yen. G-SRIF is a new generation of somatostatin, created by attaching sugar chains to somatostatin (see Figure 1). G-SRIF, co-developed by Yung Shin Pharmaceutical, is the first investigational drug on the market to improve stability in blood while maintaining the same affinity for five receptor subtypes as natural somatostatin.
To accelerate the clinical development of G-SRIF, Yung Shin Pharmaceutical has selected G-SRIF to conduct a Phase 1 clinical trial with GlyTech as the primary treatment for acromegaly, as well as preclinical trials to explore other therapeutic indications. The Phase 1 clinical trial is scheduled to begin in Japan in February 2020.
Figure :1G-SRIF