

Guarding people's health is Mr. Tien-Te Lee's lifelong vocation and the reason for Yung Shin to deal in good faith.
Mr. Tien-Te Lee’s childhood wish was to build a large garden without fences. From a pharmacy to an international pharmaceutical company, to a boundless garden that cultivates the soul, to YungShin Social Welfare Foundation that organizes volleyball tournaments to promote health, and a nursing home to implement caring to the elderly and their peace of mind, and YungShin Tien-Te Lee Biomedical Foundation to cultivate research and development talents.
Mr. Tien-Te Lee, the founder of Yung Shin Pharmaceuitcal, continues to realize his dream of guarding people's health. This aspiration is the mission that Yung Shin people continue to work hard for.
Pharmaceutical spirit: continuous pursuit of quality
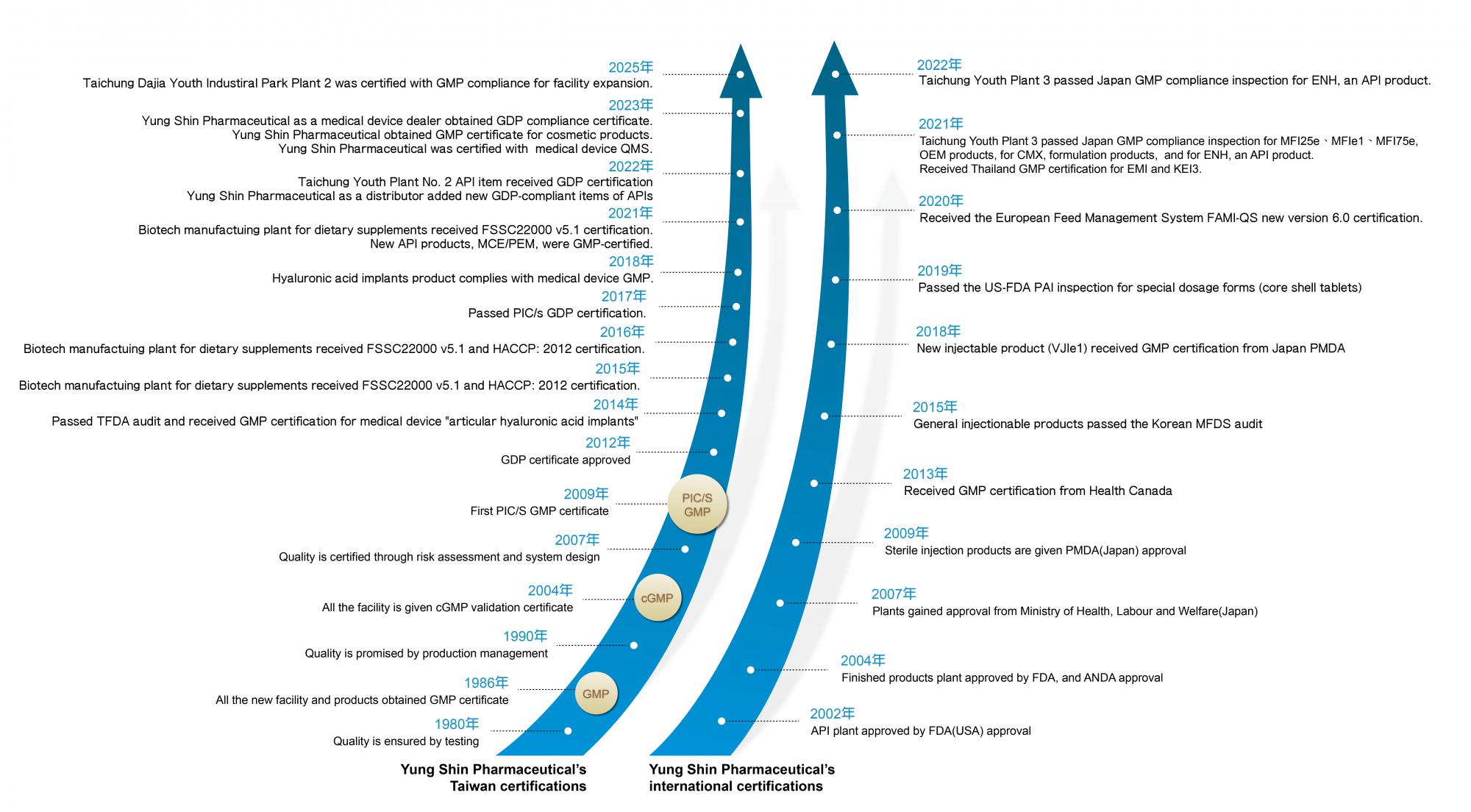
When the factory was established in 1965, Taiwan had not yet implemented Good Manufacturing Practices (GMP). Yung Shin, adhering to the GMP principles, employed accounting systems to monitor the correct execution of everything from raw materials to manufacturing processes, ensuring quality control. In 1974, the company began exporting to Southeast Asian countries and established branches.
Yung Shin pursues quality improvement and product research and development year by year, introducing sophisticated and novel testing instruments and high-efficiency production equipment, and regularly conducts self-management to ensure the effectiveness of equipment.
To better meet international pharmaceutical standards, Yung-Shin took the lead in building a new factory. In 1986, it became the first company in Taiwan to pass GMP certification for all its products at one time. In 2004, it also successfully became the first company in Taiwan to pass the cGMP three-stage validation certification for its entire factory.
Later, due to the export of our formulation products, we were the first to pass the US FDA inspection, the first pharmaceutical company in Asia’s Greater China area to export to the United States. In 2007, we obtained certification from the Ministry of Health, Labor and Welfare of Japan, and in 2009, we successfully obtained the first PIC/s GMP certification in Taiwan. Yung Shin's pursuit of quality never ends.

One unified accounting system to sustain product quality.
One unified accounting system to make product management transparent.
Since Yung Shin Pharmaceutical’s establishment, one unified accounting system records its history, which is open, transparent and traceable.
Only under the strict control of one unified accounting system can Yung Shin Pharmaceutical achieve mutual checks and balances in the management of raw materials, processes and products, without any leaks. We demand the highest product quality to make patients feel safe to our medicines.

Providing the best medicines to
improve human beings’health
Over the years, Yung Shin Pharmaceutical has been committed to its core business with the spirit of service, integrity and innovation, and has now developed into a multinational pharmaceutical and health technology group.
In the future, we will be more committed to improving the quality of pharmaceutical manufacturing and leveraging the solid foundation we have established overseas over the years to strengthen our international competitiveness and strategic plans in the global market, so as to achieve our goals of production specialization and globalization as early as possible.
To ensure a brighter future for Yung Shin Pharmaceutical, we will leverage our expertise and technology to expand our reach and realize our corporate vision of "guarding health, creating beauty, and delivering happiness." We will forge ahead with the unwavering determination to establish ourselves firmly in Taiwan, expand our horizons across Asia, and advance internationally.